 |
沪慧(上海)生物科技有限公司
订购/客服热线:400-138-0886
地址:上海市金山区亭林镇寺平南路19号3幢2938室 |
|
|
 |
原装进口试剂 |
 您的当前位置:首页 -> 产品中心 -> 原装进口试剂
您的当前位置:首页 -> 产品中心 -> 原装进口试剂
|
|
| 产品编号:87333-250G-F |
产地:sigma原装 |
| 中文名称:聚乙二醇 |
原货号:87333 |
| 英文名称:Poly(ethylene glycol) |
规格:250G |
| 分 子 式:87333 |
分 子 量:2-3天 |
| CAS# :25322-68-3 |
单位:瓶 |
| 型号: |
单价:
¥446.29.00
折扣价:¥424.00
|
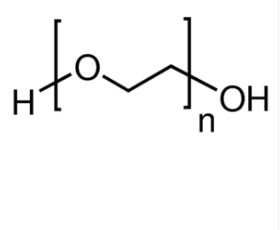
| 备注:BioUltra, 600 别名: PEG 分子式 H(OCH2CH2)nOH |
| 中文别名:424英文别名:0 |
| 危 险 品: |
危险系数: |
在线订购:   |
产品说明书:  |
|
 |
| 产品属性: |
| Product Name |
聚乙二醇,
BioUltra, 600 |
| Product Number |
87333 |
| Product Brand |
SIGMA |
| CAS Number |
25322-68-3 |
| |
| TEST |
SPECIFICATION |
| APPEARANCE (COLOR) |
Colorless or White |
| APPEARANCE (FORM) |
Liquid or Viscous Liquid or Paste |
| GEL-PERMEATION CHROM. |
CORRESPONDS TO REQUIREMENTS |
| DENSITY D20/4 |
1.115 - 1.144 |
| REFRACTIVE INDEX N20/D |
1.468 - 1.470 |
| WATER |
≤ 1.0 % |
| SULFATED ASH |
≤ 0.2 % |
| REM. ON PHYSICAL DATA |
MOLECULAR MASS(CALCULATED FROM HYDROXYL VALUE): 570 - 630 |
| INFRARED SPECTRUM |
CONFORMS TO STRUCTURE |
| VISCOSITY (ROTATION) |
150 - 190 mPas |
| VISCOSITY (CONDITIONS) |
NEAT, 20 C |
| HYDROXYL VALUE |
178 - 197 mg KOH/g |
| TRACES PEROXIDE (AS H2O2) |
≤ 20 mg/kg |
| METAL TRACE ANALYSIS (ICP) |
CORRESPONDS TO REQUIREMENTS |
| ALUMINIUM (ICP) |
≤ 5.0 mg/kg |
| BARIUM (ICP) |
≤ 5.0 mg/kg |
| BISMUTH (ICP) |
≤ 5.0 mg/kg |
| CALCIUM (ICP) |
≤ 10 mg/kg |
| CADMIUM (ICP) |
≤ 5.0 mg/kg |
| COBALT (ICP) |
≤ 5.0 mg/kg |
| CHROMIUM (ICP) |
≤ 5.0 mg/kg |
| COPPER (ICP) |
≤ 5.0 mg/kg |
| IRON (ICP) |
≤ 5.0 mg/kg |
| POTASSIUM (ICP) |
≤ 500 mg/kg |
| LITHIUM (ICP) |
≤ 5.0 mg/kg |
| MAGNESIUM (ICP) |
≤ 5.0 mg/kg |
| MANGANESE (ICP) |
≤ 5.0 mg/kg |
| MOLYBDENUM (ICP) |
≤ 5.0 mg/kg |
| SODIUM (ICP) |
≤ 200 mg/kg |
| NICKEL (ICP) |
≤ 5.0 mg/kg |
| LEAD (ICP) |
≤ 5.0 mg/kg |
| STRONTIUM (ICP) |
≤ 5.0 mg/kg |
| ZINC (ICP) |
≤ 5.0 mg/kg |
| ARSENIC TRACES (MHS-AAS) |
≤ 0.1 mg/kg |
| TOTAL SULFUR AS SO4 (ICP) |
≤ 50 mg/kg |
| CHLORIDE (CL) |
≤ 50 mg/kg |
| SOLUBILITY (METHOD) |
1.500 G IN 30 ML H2O |
| APPEARANCE (SOLUTION) |
CLEAR COLORLESS |
| PH (SOLUTION) |
5.5 - 7.0 |
| RESIDUE (FILTER TEST) |
NO RESIDUE |
| UV - ABS. AT 260 NM |
≤ 0.06 |
| UV - ABS. AT 280 NM |
≤ 0.03 |
| RECOMMENDED RETEST PERIOD |
42 MONTHS |
|
|
|

